Go Back:
Home / Gas Laws / Combined Gas Law /
Combined Gas Law Practice Problems (no moles)
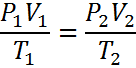 or
or 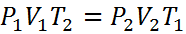
This site will produce an unlimited number of Combined Gas Law Law practice problems (no moles).
You must enter your name and select a teacher before you are allowed to check any answers. Select the
teacher 'Guest Student' if you are not from Haltom High School.
Total: 0,Tried: 0, Correct: 0
August 01 2025, 22:27:43 GMT
Ver 01.01.03 April 17, 2016 - by G. W. Franz
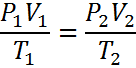 or
or