The Combined Gas Law
NO LESSON YET,
but the practice problems work.
Push the Green button on the right.
The combined gas law can be seen as a combination of three other gas laws, Charles' Law, Boyle's Law and Gay-Lussac's Law. It is generally written two ways:
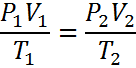 |
|
| Equation #1 | Equation #2 |
Both ways have advantages - Equation #1 has the benefit of having all if the inital conditions on the left side (1) and the final conditions on the right (2). Its problem is in solving for T1 and T2. Equation #2 has some 1's and 2's on each side. The big advantage of Equation #2 is that is is very easy to rearrange. Just circle the variable you want to keep and then move all of the others to the other side, under the other variables.
For example: Solving for T1 is easy if you start with Equation #2
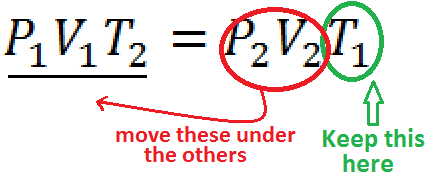 |
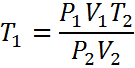 |
| Just circle the one you want to keep, and move the others underneath the other side | Presto - You're done |
All you have to do now is substitute your known variables to get the unknown.