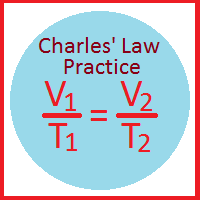
Charles' Law of Volumes
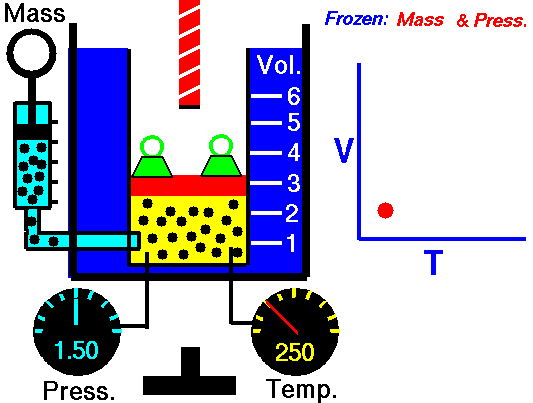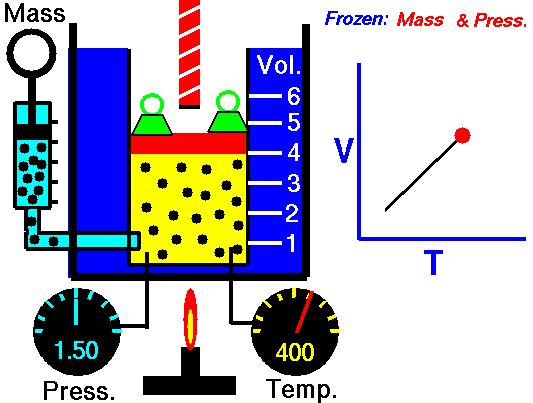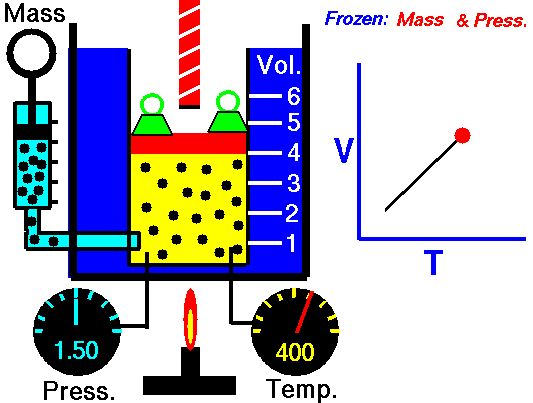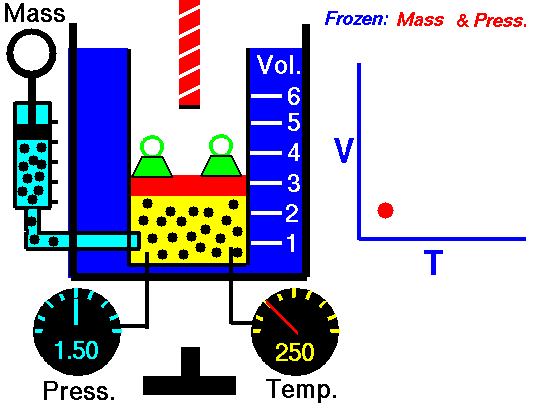
Charles' Law states that the volume of a gas is directly related (directly proportional) to its absolute temperature. In other words, as the temperature goes up, so does the volume. As the temperature decreases, so does the volume. This relationship holds at constant pressure, which means that all measurements of volume and temperature occur at the same pressure.
Observe the animation to the right, and see how adding heat causes an increase in volume when the pressure is constant. That the pressure is constant is indicated by the green weights holding down the lid.Charles' Law can be expressed in three ways:
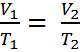 |
||
| Equation #1 | Equation #2 | Equation #3 |
The first equation leads to the second and third. Here is how - Given that
V is the volume of the gas
T is the absolute temperature of the gas (measured in kelvin).
k is a constant (which depends on the units of V & T)
Pressure in this relationship is a constant, so is not part of the equation
If the ratio (or fraction) of V and T is always a constant, then we can use an initial pressure and temperature, V1 and T1 to solve for volume or temperature at another set of conditions, V2 or T2. This is because if V1/T1 = k, it must also be true that,
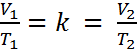
We can remove “k” and we get Equation #2. A little rearranging give us Equation #3.
It is important to note that you cannot solve this equation with temperatures in °C. You must convert to absolute temperature, or Kelvin (K). This is easy enough to do because, K = °C + 273. Also, volumes can be measured in any units, but whatever they are, both volumes must be in the same units.
Most students find the form of Equation #2 easiest to use. Consider the following word problem:
A gas with a volume of 2.5 Liters and a temperature of 350 Kelvin is cooled to a temperature of 280 Kelvin. What is the new volume?
First we identify the variables, V1=2.5 L, T1=350K, T2 = 280 K and V2 is unknown. The temperatures absolutely must be in Kelvin. If the temperatures are in °C, simply add 273° to get the temperature in Kelvin. Now, we substitute into Equation #2 and get:
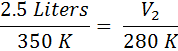
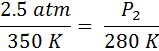
From here, we just cross-multiply the two circled numbers and then divide by the third
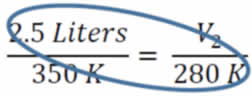
V2 = (280K x 2.5 L) / 350K = 2.0 L. You can subsitute and cross multiply to solve for any of the four variables.