Avogadro's Law
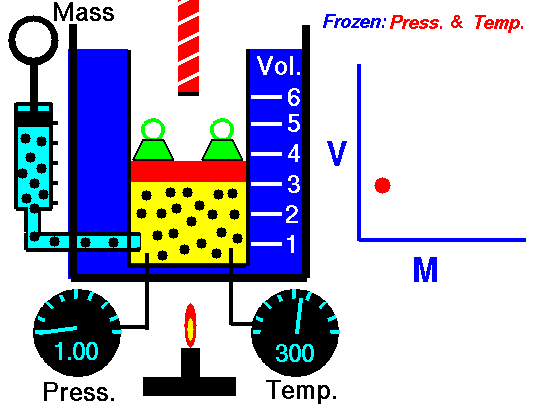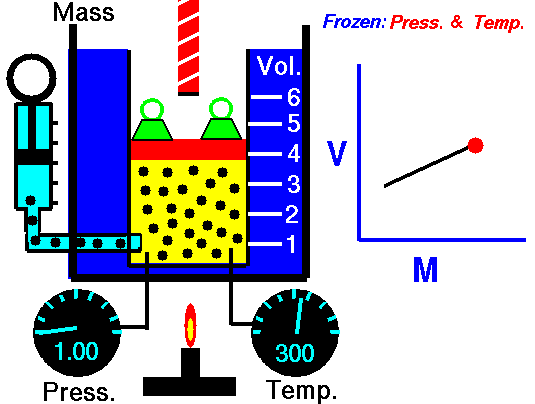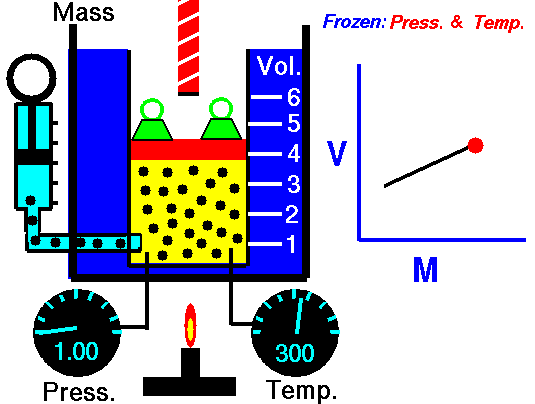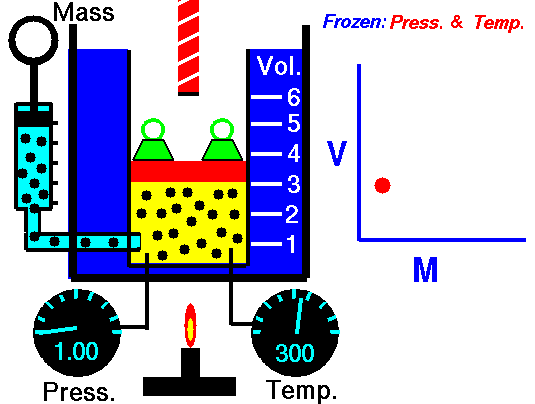
Avogadro's Law (also called Avogadro's hypothesis, or Avogadro's Principle) states that equal volumes of gases at the same temperature and pressure have the same number of molecules of gas. In other words, in an ideal gas, the number of volume of a gas is directly proportional to the number of particles of gas in that volume as long as the temperature and pressure are the same. The molar mass of the gas does not matter. In fact, Avogadro's Principle can be used to determine the molar mass of an unknown gas, since the number of particles can be calculated.
Avogadro's Law can be expressed in three ways:
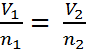 |
||
| Equation #1 | Equation #2 | Equation #3 |
The first equation leads to the second and third. Here is the process. If
![]() where,
where,
V is the volume of the gas,
n is the number of moles of particles of gas (usually molecules), and
k is a constant (which depends on the units of P & T)
then, since the ratio (or fraction) of V and n is always a constant, we can use an initial volume and number of moles, V1 and n1 to solve for volume or moles at another set of conditions, V2 or n2. This is because if V1/n1 = k, it must also be true that,
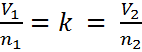
We can remove “k” and we get Equation #2. A little rearranging can also give us Equation #3; however, most students find the form of Equation #2 easiest to use. Consider the following word problem:
At a constant pressure and temperature a gas which contains 0.350 moles occupies a volume of 11.5 Liters. If another 0.150 moles are added, what will be the new volume?
First we identify the variables, V1=11.5 L, n1=0.350 moles, n2 = (0.350 + 0.150) = 0.500 moles, and V2 is unknown. The temperature and pressure are unknown, but not important as long as they are constant. Now, we substitute into Equation #2 and get:
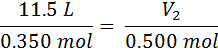
From here, we just cross-multiply the two circled numbers and then divide by the third
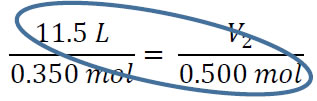
V2 = (11.5 L x 0.500 mol) / 0.350 = 16.4 L. You can subsitute and cross multiply to solve for any of the four variables as long as temperature and pressure are constant.